Background to the PROTECT Survey
The introduction of oral PrEP in Europe has been challenging. Introduction was slow, not synchronized between countries, which led to both knowledge gaps and informal procurement of early adopters. Access was and remains limited due to demonstration projects approaches, or restricted national programs, leading to a continued patchwork situation of PrEP access in Europe.


There are unmet needs in terms of access to HIV prevention tools among communities impacted by HIV. Additional ways to access PrEP can facilitate increased PrEP utilisation (uptake, adherence and persistence) among those currently unwilling or unable to use oral TDF/FTC as PrEP.
The introduction of a long acting as a new PrEP modality to the current PrEP landscape, which currently comprises oral PrEP only, is expected to reduce HIV transmission. Having more people protected from HIV supports the progress towards the UNAIDS vision (UNAIDS, 2014) of achieving zero new HIV infections and helps to reduce the substantial economic, clinical and humanistic burden associated with new HIV diagnoses in Europe. Various national action plans in Europe, corporate analyses and research findings support increasing access to evidence based HIV prevention strategies such as PrEP to help eliminate HIV.
This survey explores PrEP uptake intentions across Europe
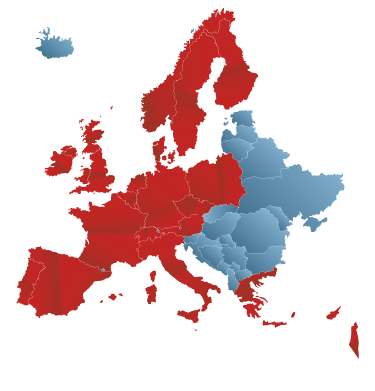
Austria, Belgium, Cyprus, Czech Republic, Denmark, Finland, France, Germany, Grece, Ireland, Israel, Italy, Luxembourg, Netherlands, Norway, Poland, Portugal, Spain, Sweden, Switzerland, UK.
To date there is little data available on interest and intention to use a long acting injectable PrEP formulation in Europe and data from other contexts is not applicable to the European context due to different demographics, psycho-social determinants or healthcare systems.
Data in this survey was collected via online convenience sampling. The survey sought to understand who could benefit from long acting injectable PrEP in this diverse region and how these benefits are perceived. It also investigated the motivations for long acting injectable PrEP uptake, oral PrEP usage patterns, unmet needs related to oral PrEP use, and their correlation with interest in long acting injectable PrEP and preferred access pathways


The approach taken targeted multiple populations of interest to explore long acting injectable PrEP uptake more comprehensively. Men who have sex with men, transgender males and females, heterosexual cisgender men and women who have sex with men, and people living with HIV were all engaged via tailored online surveys. The multi country approach allowed for both inter- and intra-country comparison of data.
Given the potential for individuals to use long acting injectable PrEP in the future, an investigation of the determinants and needs of individuals for long acting injectable PrEP use in Europe is paramount to supporting its additional benefits recognition and uptake.
This survey is partially funded by ViiV healthcare.
The funder is not responsible for the promotion activities related to the project, nor the analysis or interpretation of data.