News
EVENT
The PROTECT team is running a pub quiz in Glasgow on long acting injectable PrEP on Tuesday 13 November 2024. See the flyer at the link below for more details:
CONFERENCE NEWS
The PROTECT team presented the following presentations at the HIV Glasgow Conference in November 2024:
- Presentation – Differences in oral PrEP use patterns and intention to use long acting regimens among MSM between governmental and non governmental PrEP provision pathways in 20 European countries: A latent class analysis
- Poster – Differences in oral PrEP use patterns and intention to use long-acting regimens among MSM between governmental and non-governmental PrEP provision pathways in 20 European countries: A latent class analysis
- Preparing for long-acting PrEP delivery: Provider preferences for the provision of long-acting PrEP differ between MSM and heterosexual individuals in Europe
- Intention to use long-acting PrEP among 4960 heterosexual women and men in 20 European countries — results from the PROTECT survey
The PROTECT team presented the following presentations at the Fast Track Cities Conference in Paris in October 2024:
Awareness and use of oral PrEP and the intention to use long-acting PrEP in 6 European Fast Track Cities (FTC) & 1 capital city – a geo-spatial gap analysis based on data from the PROTECT survey
“A country is more than its capital” – a geo-spatial gap analysis of oral PrEP awareness, eligibility and use, and intention to use long-acting PrEP in France, based on PROTECT survey data
CONFERENCE NEWS
The PROTECT team is presenting analysis of PROTECT survey data at the 25th International AIDS Conference in Munich. The following in person poster presentations will be given:
- Intention to use long-acting PrEP among MSM in Europe – results from the PROTECT survey from Spain, Italy, Germany, France and the United Kingdom. K. Jonas , J. Kolstee , H. Wang , A. Adriaque Lozano , J.C. Croce , M. Schroeder , A. Appiah , C. Brown , A. Milinkovic , J. Scherzer , H. Zimmermann
- Interest and intention to use long-acting injectable PrEP for HIV (LA-PrEP) among MSM and trans people in the Netherlands. J. Kolstee , H. Wang , H. Zimmermann , A. Adriaque Lozano , M. Schroeder , A. Appiah , C. Brown , K. Jonas
- Preferences for the provision of oral and injectable PrEP among MSM and transgender persons who discontinued oral PrEP in Europe. H. Zimmermann , H. Wang , J. Kolstee , A. Adriaque Lozano , M. Schroeder , A. Appiah , C. Brown , J. Scherzer , K. Jonas
- Exploring the Relationship Between Comprehensive Sexual Health Prevention Measures and the Intent to Use Long-Acting PrEP among MSM. A. Adriaque Lozano , J. Kolstee , H. Wang , H. Zimmermann , M. Schroeder , A. Appliah , C. Brown , A. Milinkovic , K. Jonas
- Mapping PrEP use cascades in the Netherlands under the internalised homonegativity “storm” and sexual self-efficacy “sunshine”: Where do MSM need an umbrella and where do they need sunglasses? Haoyi Wang, Johann Kolstee, Hanne M.L. Zimmermann, Kai J. Jonas
- A psycho-social weather report in the Netherlands: Mapping the internalised homonegativity “storms” and the sexual self-efficacy “sunshine” among MSM and their ecological associations with HIV care cascade. Haoyi Wang, Hanne M.L. Zimmermann, Johann Kolstee, Kai J. Jonas
CONFERENCE NEWS

The PROTECT team is hosting a satellite symposium at the 25th International AIDS Conference in Munich.
Start your conference week with a focus on LA-PREP with our session on Monday 22 July at 0730hrs!
For more information see below.
Title: Fitting my needs – HIV prevention in the age of biomedical alternatives (oral and long-acting PrEP, TasP)
The satellite focuses on the topic of biomedical HIV prevention choices that are now available: oral/long-acting PrEP and treatment as prevention (TasP). Experiences with TasP, and HIV stigma are being discussed jointly with data on user experience of oral and long-acting PrEP (e.g. SEARCH), as well as intention to use long-acting PrEP data (e.g. the European PROTECT survey w/ 20.000 participants). We will critically investigate tailored prevention potentials, unmet needs and access pathways and their impact on ending the HIV epidemic. As speakers, the following researchers covering the contexts in Europe (Kai Jonas, Johann Kolstee, Hanne Zimmermann, Haoyi Wang, The Netherlands), Southeast Asia (Natthakhet Yaemim, Thailand), Australasia (Martin Holt, Australia), Africa (Jane Kabami, Uganda), and the USA (Katie Biello) will contribute. In a panel, we are reflecting on the results with key populations represented by the following panelists Amanita Calderon-Cifuentes (Transgender Europe – TGEU), Juddy Otti (Africa Advocacy Foundation), Alex Schneider (Life4me+), Samira Hakim (Trans United Europe).
Survey Update
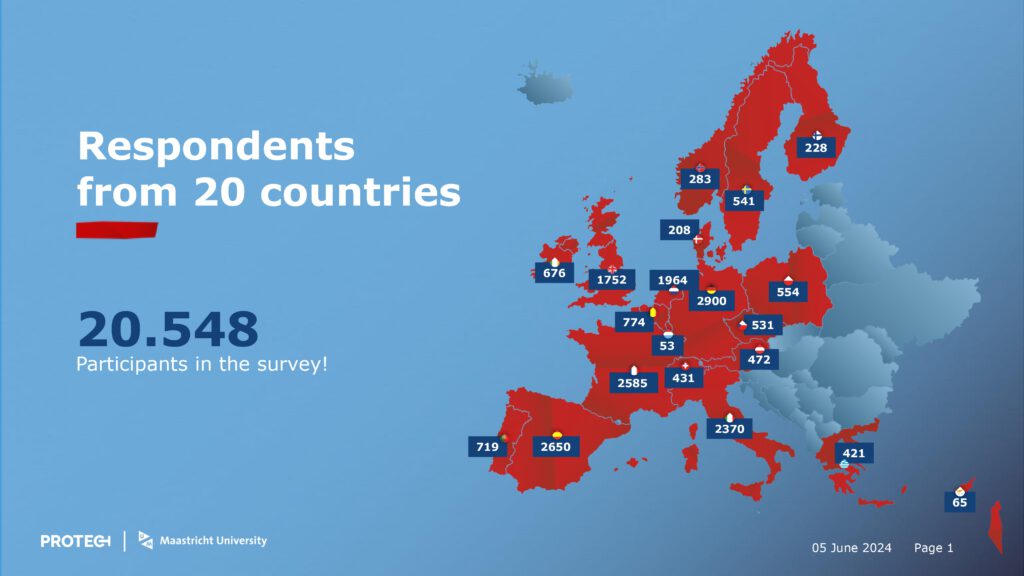
The PROTECT Survey has officially stopped collecting data. We have reached our data collection targets and the PROTECT Survey team would like to thank all of the people who have participated.
SURVEY UPDATE
PROTECT SURVEY PROTOCOL
The PROTECT Survey Protocol was presented at the Fast Track Cities Conference in Amsterdam in September 2023. For more information, please see attached doc (PDF).
19/09/23
Availability update
European Commission authorises ViiV Healthcare’s Apretude (cabotegravir long-acting and tablets) for HIV prevention
The European Commission (EC) has authorised Apretude (cabotegravir long-acting [LA] injectable and tablets) for HIV prevention. Cabotegravir injection is indicated in combination with safer sex practices for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV-1 in high-risk adults and adolescents (at least 12 years of age), weighing at least 35 kg. The availability of cabotegravir LA for PrEP will differ from country to country across the EU. ViiV Healthcare are committed to working with respective regulatory authorities, governments, payors and other stakeholders to understand the local needs and make this product available to people who can benefit from this innovative prevention option.
26/07/23
SURVEY UPDATE
The PROTECT survey will be available to complete in a select number of countries in Europe from October 2023.
Watch this space for more updates on the exact launch date and which countries will be participating first.
20/07/23
Journal article
Determinants of PrEP Uptake, Intention and Awareness in the Netherlands: A Socio-Spatial Analysis
Learn more about research into long acting injectable PrEP. Explore a recent article that explores determinants of PrEP Uptake, Intention and Awareness in the Netherlands.
18/07/23
Journal article
Spatio-temporal changes in pre-exposure prophylaxis uptake among MSM in mainland France between 2016 and 2021
This study used national pharmaco-epidemiology surveillance data and regional MSM population estimations to model the spatio-temporal distribution of PrEP uptake among MSM in France 2016–2021 to identify marginalized MSM at risk for HIV and increase their PrEP uptake.